Abstract
Introduction: The mammalian target of rapamycin (mTOR) functions as a critical catalytic core in two distinct intracellular complexes: the rapamycin-sensitive mTORC1 and the rapamycin-insensitive mTORC2. The latter is capable of activating Akt through phosphorylation at the Ser473 residue. Constitutive activation of Akt is identified in up to 70% of acute myeloid leukemia (AML) patients and has been associated with an adverse impact on survival. Akt activation also mediates, at least in part, the leukemogenic effects of activating FLT3 internal tandem duplication (ITD) mutations, which in turn lead to activation of mTORC1. Mitogen-activated protein kinase-associated protein 1 (MAPKAP1 or Sin1) is required for mTORC2 integrity and activation and is also required for phosphorylation and activation of Akt. In addition, the pleckstrin homology (PH) domain of MAPKAP1 directly binds the PI3K product PtdIns P3 to promote mTORC2 kinase activation and membrane localization, thereby revealing a mechanistic link between PI3K and mTORC2. However, the role of MAPKAP1 in AML pathogenesis and its impact on clinical outcomes are as of yet unknown.
Methods: The in vitro system included 5 AML cell lines (2 FLT3 -mutated and 3 FLT3 -unmutated) and 6 BA/F3 (murine pro-B cells) stable clones bearing specific FLT3 mutations. Experiments were performed using transient transfections with siRNA or plasmids, pharmacologic studies, Western blot analysis, q-RT-PCR, flow cytometry, immunofluorescence, confocal microscopy and proteomics. The patient cohort included 224 adult AML patients diagnosed and treated at The University of Texas MD Anderson Cancer Center (Houston, TX) and the National University Hospital (Singapore) with available cytogenetics and mutation status data. Potential associations of MAPKAP1 with clinical outcomes were evaluated using mRNA expression data from the The Cancer Genome Atlas (TCGA) AML dataset and protein expression data assessed by immunohistochemistry on bone marrow biopsy specimens from patients in our study group using a monoclonal anti-MAPKAP1 antibody (Cell Signaling). Overall survival (OS) was defined as time from AML diagnosis to death or last follow-up. Event-free survival (EFS) was defined as time from AML diagnosis to relapse, death, or last follow-up. Survival analysis was based on the Kaplan-Meier method using the Log-rank test.
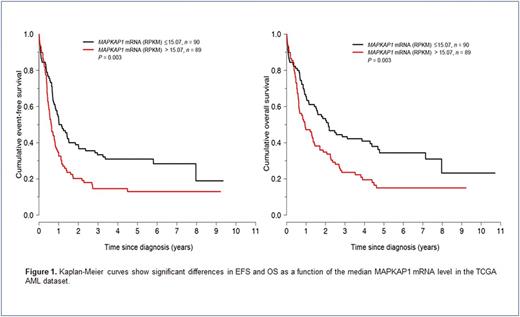
Results: MAPKAP1 is frequently expressed in AML cells and regulated by STAT3, which is predominantly phosphorylated at Ser727 residue in AML cell lines and patient samples. Inhibition of STAT3 activity by pharmacologic agents, dominant negative constructs, or STAT3 gene silencing leads to decreased MAPKAP1 levels and MTORC2 activity associated with Akt dephosphorylation. In silico analysis reveals 4 potential STAT3 binding sites on the MAPKAP1 gene promoter suggesting transcriptional regulation. Knocking down the MAPKAP1 gene in AML cell lines results in Akt deactivation associated with decreased cell proliferation, and these effects are mediated by cell cycle regulators. MAPKAP1 also confers resistance to sorafenib in vitro, as MAPKAP1 is shown to be upregulated and more active in BA/F3 clones bearing sorafenib-resistant FLT3 mutations. Conversely, STAT3 or MAPKAP1 inhibition sensitizes resistant BA/F3 clones to sorafenib treatment. Using the TCGA dataset, high MAPKAP1 mRNA levels are significantly associated with poorer EFS and OS (Figure 1); these findings were independent of AML risk group or the presence of FLT3 or NPM1 mutations. Similarly, high MAPKAP1 protein levels assessed by immunohistochemistry on bone marrow specimens prior to treatment correlate with adverse clinical outcomes in a cohort of AML patients treated with equivalent regimens.
Conclusions: MAPKAP1 is involved in AML pathogenesis, likely through a STAT3-MTORC2-Akt crosstalk mechanism, and may represent a novel target of investigational therapy. MAPKAP1 mRNA and protein levels are significantly associated with clinical outcomes in AML.
_
Khoury: Pfizer: Research Funding; Angle: Research Funding; Stemline Therapeutics: Research Funding; Kiromics: Research Funding. Kantarjian: Delta-Fly Pharma: Research Funding; Amgen: Research Funding; ARIAD: Research Funding; Bristol-Meyers Squibb: Research Funding; Pfizer: Research Funding; Novartis: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.